|
|
|
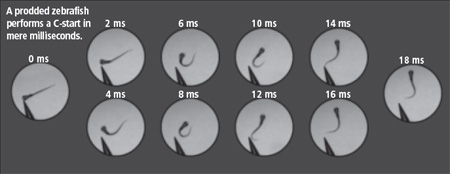
Spherical eyes bulging, a six-day-old zebrafish darts around its petri-dish pool. A tap to its tiny head with a needle-like prod sets off a reaction so quick that it’s over within mere milliseconds: the fish bends into a C and then swims off, threat evaded. Another poke brings a different response: the fish shifts into an S. Melina Hale, assistant professor of organismal biology and anatomy, studies those movements in hopes of identifying the nerve cells that regulate fish locomotion. Hale, PhD’98, focuses on brain and spinal-chord activity in motor control systems that behave similarly to those in higher vertebrates, including humans. With fish, “there are a lot of analogies to how we move,” she explains from her anatomy lab office. Related mechanisms, for example, get their tails wagging and our legs pumping, she says. Because of the biological parallels, her work could one day lead to cures for neurodegenerative disorders that diminish mobility, such as Amyotrophic Lateral Sclerosis (ALS), commonly called Lou Gehrig’s disease.
“There is just so much to learn about the brain and the spinal chord,” she says in a video interview posted on Research at Chicago, a University Web site. “We know such a small component of what is out there—not even talking about these broader issues of consciousness—but just circuit breaking, figuring out how these neural circuits, how these cells, are connected to allow them to function.” Hale’s own attempts at circuit breaking—tracking down the nerve cells responsible for motor control—build on her doctoral research on fish swimming. “Looking at the development of locomotor behavior in an evolutionary context,” she says, “I became more and more interested in how the behavior was controlled neurally.” To investigate the brain–behavior connection she turned her attention to startle response, the system that steers prey’s flight from predators and other threatening stimuli. “When someone sneaks up behind you,” she explains, “we tend to startle. It’s a fast involuntary response. In fishes it’s an escape movement. They will bend away from the stimuli into a C-shape and then swim away.” The C-start, as it’s known, is easily seen in live, larval zebrafish, whose transparency makes plain their nerve cells. Injecting those cells with fluorescent dyes, Hale films five- to six-day-old zebrafish under a microscope and a high-speed video camera. The equipment records the fish’s movement and neural activity as she gently pokes its body, triggering a C-start. “The basics,” she notes, “have been studied for well over 100 years,” and biologists have shown that Mauthner cells, a pair of large neurons located in the hindbrain, direct the response. “Until just a few years ago we thought the C-start was the startle of fishes.” In late 2000, however, “we found there is an alternative startle,” the S-start, which involves “a different population of cells in the brain.” Researchers had previously observed the S-start, also named for its shape, in many species, she says, but assumed it was essentially a variation on the C-start, with water pushing the tail to create the S formation. “It seemed something more was going on,” recalls Hale, adding that her own behavioral work made her question the earlier hypothesis. “That sort of opened up my thinking”—and propelled her to begin examining the S-start as well. Implanting electrodes in fish, she documented a change in muscle activity between the starts. “It told us that the neural components had to be different,” she says. In the May 2002 Journal of Experimental Biology she reported that the two responses rely on separate neural mechanisms. Since then she’s been trying to break the S-start circuit so she can compare it with the C-start’s. The systems are “driving a very similar behavior and both are likely to be very simple,” she says. But because the S-start coordinates muscle activity on both ends of the fish, hence its shape, its code likely includes “different features that should provide us with some additional information on circuit organization.” To narrow her search for the S-start circuit she kills individual neurons with a laser and contrasts the fish’s behavior with and without them, sizing up each one’s role in startle response. Thanks to long-standing anatomic work, she’s concentrating on 50 to 60 cells in the hindbrain, including the Mauthners, that send signals down the spinal chord. Rounding out her understanding of vertebrate motor control, Hale also focuses on the spinal chord. Again using larval zebrafish, fluorescent dyes, and microscopy, she explores how spinal-neuron populations work together during startle response and routine, rhythmic swimming. “It’s a lot of new territory,” she says, “and really setting the foundations for future work,” including clinical trials on therapies for spinal-chord injuries and illnesses. One project she has under way involves developing a mutant line of zebrafish with the same genetic defect that causes ALS, a progressive neurodegenerative disease. “A lot of the changes in the deterioration of nerve cells occur before the disease is diagnosed because the nervous system is good at compensating for the loss,” she explains. Pursuing what happens early on in the modified fish, she next plans to use time-lapse imaging to chart the disease’s beginning stages. “The broad goal for my lab,” Hale
sums up, “is to understand the relationship between the brain
and spinal chord and nerve cells and circuits they’re organized
into.” Back in the lab and seated at the microscope, she observes
another set of flips and flops—patiently fishing for new clues.—M.L. |
|
phone: 773/702-2163 | fax: 773/702-8836 | uchicago-magazine@uchicago.edu

 Investigations
Investigations