Chief of staph
Pediatrician Robert Daum battles deadly resistant bacteria
By Lydialyle Gibson
Photography by Susan Boyle-Vavra
When pediatrician Robert Daum talks about one day being able to dismantle a MRSA bacterium’s defenses and destroy it with antibiotics, he holds his hands in front of him and shakes them slightly, as if the germ were a person he might throttle. “We’re working on it,” he says, “but it’s slow going.”
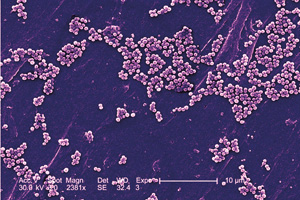 Usually MRSA cells, seen here through a scanning electron microscope, remain on the skin, but when the infection goes deeper, Daum says, “all hell breaks loose.”
Usually MRSA cells, seen here through a scanning electron microscope, remain on the skin, but when the infection goes deeper, Daum says, “all hell breaks loose.”
For more than a decade, Daum has been fighting a germ that long ago learned to fight back. Methicillin-resistent Staphylococcus aureus—MRSA—is a relatively recent strain of an ancient human pathogen. After penicillin emerged in the early 20th century, Staphylococcus aureus adapted, producing an enzyme that could defeat it. Other antibiotics followed, among them methicillin, first introduced in 1959. By 1961 a British bacteriologist was reporting the existence of a new staph strain: MRSA.
For decades the germ—resistant not only to methicillin but also to many other antibiotics—was confined to hospitals and clinics, where constant exposure to drugs drove immunity. Chronically ill patients, in and out of the hospital often, were susceptible, but generally not people in the surrounding community. “That was the way the world looked,” Daum says, “until a decade ago.”
In 1996 he and then-Chicago pediatrician Betsy Herold noticed a sharp rise in the number of children showing up at the hospital with resistant infections. At first they wondered if feral MRSA strains had escaped from hospitals into the community, but they soon realized they were dealing with a completely different strain they called community-associated MRSA. In 1998 they published their findings in the Journal of the American Medical Association. “Even now we don’t know a lot about where the community-associated MRSA came from,” Daum says, “except that, in retrospect, it was seen periodically before. Our best guess is that, one, it acquired the gene to become resistant, and, two, it acquired something—we still don’t know what—to become hypervirulent.” Today, Daum estimates, 65 percent of MRSA infections are community associated.
Most MRSA infections stay on the skin, causing boils and red, swollen abscesses. But when the bug invades deeper tissue, “all hell breaks loose,” Daum says. The germ can attack bloodstream, liver, bone. Particularly dangerous is necrotizing pneumonia, which destroys the lungs. A few antibiotics remain potent against MRSA, but they have to be administered quickly—infected children can die within a day or two.
With an expanding roster of collaborators at Chicago and elsewhere, Daum is involved in a dozen research projects aimed at deciphering and defeating MRSA (and a Web site chronicles their efforts). There are studies into the bacterium’s genetic profile and the genetic particularities of those most vulnerable to it. A treatment study with Medical Center patients compares the effectiveness of existing antibiotics. One project sends investigators to the homes of MRSA patients to culture everyone in the household and ask “risk-factor” questions about how often they wash their clothes or touch other people, whether they’ve had MRSA before or been exposed to HIV. The team also tests commonly handled objects, like doorknobs and remote controls.
Another study, under way since January, follows prisoners in the Dallas County Jail for three years. “Jail is perfect for MRSA,” Daum says: prisoners seldom bathe, because showering can be dangerous, and they wear the same clothes day after day, because once they find a suit that fits, they’re reluctant to surrender it to the laundry. What’s more, Daum says, bored inmates tend to pick each other’s boils. In that environment, MRSA runs rampant. Daum and his colleagues will determine how many detainees carry MRSA on their skin or in their noses and how many carriers become infected, which MRSA strains the jail has, and whether bathing with chlorhexidine wipes cuts the contagion rate. The results could improve prevention in other public places.
Daum’s favorite study is the one he hopes will leave MRSA defenseless. “Bacteria may seem inert, but they’re sampling their environment all the time,” he says. Proteins sticking out from the cell detect threats to the cell wall and send a signal that turns on the genes to produce a response. “The most feared stress of all is an antibiotic in their midst,” Daum says. In the lab, he’s working on a way to disrupt MRSA’s sensor protein, which would short-circuit the system. The signal would never get sent, the genes would never fire, and the germ would never arm itself. “Then you can sneak up on it,” he says, with almost any antibiotic. Daum and his colleagues work with the Joint Craig Venter Institute (whose researchers sequenced the human genome) to sequence MRSA strains to find out which genes light up when Daum manipulates the threat-signal proteins in the lab. Then they compare them to unaltered MRSA proteins. The process requires a bit of hunting and pecking, trial and error, but Daum thinks it will work. “We just have to find the drug that knocks out the system.”