Physics
for breakfast
>> When
physicist Sidney Nagel sits down to breakfast each morning, he's
also doing research-on disordered, nonlinear phenomena. His observations
could be a manufacturer's meal ticket.
 Breakfast
is a messy meal. It involves sticky stuff that drips, like honey
or syrup, intended for pancakes but destined for your elbow, gritty
granules of sugar that cascade off your quivering spoon, and hot
caffeinated liquids that slosh, spill, and stain. Don't blame
yourself. Breakfast food, we now know, does not behave properly.
Coffee and a roll, hash browns, over-easy eggs, sausage, and a
side of toast: these are nonlinear and disordered phenomena, appearing
in macroscopic systems far from equilibrium.
Breakfast
is a messy meal. It involves sticky stuff that drips, like honey
or syrup, intended for pancakes but destined for your elbow, gritty
granules of sugar that cascade off your quivering spoon, and hot
caffeinated liquids that slosh, spill, and stain. Don't blame
yourself. Breakfast food, we now know, does not behave properly.
Coffee and a roll, hash browns, over-easy eggs, sausage, and a
side of toast: these are nonlinear and disordered phenomena, appearing
in macroscopic systems far from equilibrium.
Fortunately,
Sidney Nagel, the Stein Frieler distinguished service professor
of physics, studies nonlinear and disordered phenomena in macroscopic
systems far from equilibrium-which is tech talk for the scrambled,
agglomerated, unpredictable world we can see and smell and swallow.
For ten years Nagel and his students and colleagues have been
meditating on the mechanics of this messy morning meal and have
begun to unravel the problematic physics of breakfast: why syrups
drip, granules avalanche, and coffee forms distinctive stains.
Most recently, they have explained-but not corrected-the inequities
caused by Brazil nuts.
"These
are not just toy problems to hone our skills," says Nagel.
"They present significant questions that are tremendously
difficult to understand on their own." All of his findings,
he adds, "surprise me. In no case has nature arranged things
in the ways one might have expected."
Modern
physics is usually done at the extremes, asking questions about
the imponderably small or the unimaginably vast. Nagel wants to
grasp the mysteries that lurk in the ponderable, the imaginable,
the pourable, spillable, and edible.
"How
can we maintain that we are inquisitive about the world,"
he asks, "and yet remain unmoved by the omnipresent occurrences
that disturb our daily existence?" These daily disturbances
are so ubiquitous that they dominate a wide variety of industrial
applications, he insists, from how particles pass through pipes
to how paint dries. As it turns out, most of the unanticipated
answers to his previously unasked questions have practical applications
that go well beyond the breakfast table. Many technological dilemmas,
he says, could be solved if only we "knew more about the
physics staring at us each morning."
Drip
Trickle
Slosh
Crunch
Drip
Part of the problem is your sweet tooth. The sweeter the breakfast
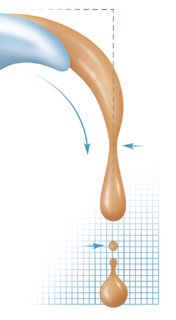
syrup, the stickier-and messier. Around 1990 Nagel began investigating
the flow and fissioning of fluids, or what happens when you pour
some water and then stop. As the flow slows, the fluid separates
into drops, but in ways that are "much more exciting"
than his preconceived ideas. High-speed photographs have revealed
a series of unanticipated geometric shapes just before and after
the "snapoff" point.
Honey
and syrup, however, are far more viscous than water; they flow
slower and appear tenaciously unwilling to let go. The more sugar
the fluid contains, the longer the connecting neck from source
to drop. Viscous liquids, Nagel has discovered, produce a series
of necks, each smaller and thinner than the last as they slowly
stretch. Indeed, Nagel notes, in an observation that may change
how ink-jet printers work or how industrial coatings are applied,
"We believe that this neck-forming-neck cascade goes on ad
infinitum until breakup."
No
wonder it gets everywhere.
 Trickle
Trickle
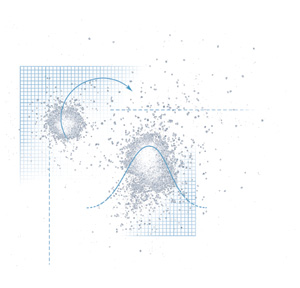
Granular materials, like sugar, oats, or coffee grounds, also
behave in "dangerous, obstinate, and unpredictable ways,"
says Nagel. Grain silos, for example, are prone to collapse because
of the unpredictable pressures created by flowing particles. Closer
to home, the pyramid of sugar on a teaspoon may seem stable at
first, but the slightest tremor on the trip from sugar bowl to
coffee cup can trigger a quiet avalanche. The problem is not tenacity
but density. For the simplest example, perfectly round particles,
density-how tightly the particles pack together-can vary by as
much as 15 percent, depending on how the grains settle into place.
Packed grains are fairly stable, but the slightest trembling enables
the grains near the surface to unpack and dilate slightly-allowing
them to flow, off the spoon and onto the table. Where they bounce
is another matter.If
this distresses you, don't take a powder. Pharmaceutical companies
face the same difficulties transporting substances that sometimes
flow like water and other times jam like cement. "It is surprisingly
difficult," admits Nagel, "to produce a uniform mixture
of powders which have different sizes, shapes, or surface properties."
Slosh
Coffee also behaves in ways that mock intuition, not so much when
guzzled as when spilled. Spills, it has long been observed, are
thickest at the center, but the stains concentrate at the edge.
A thorough investigator, Nagel has shown that this phenomenon
occurs with almost any beverage, with or without caffeine, on
most hard surfaces, even when dried upside down (in case you slosh
something-such as paint-on your ceiling). In the process, he realized
that the key was the pattern not of the spill but of evaporation,
which occurs more rapidly at the periphery, where slightly more
surface is exposed. As the water evaporates, it deposits dissolved
coffee particles underneath. Then the remaining fluid flows out
from the center to the edge, where it evaporates, forming a neat
outline of a messy spill.
Crunch
Science
marches on. In the November 15 issue of Nature, Nagel and colleagues
pointed out flaws in previous attempts to understand the "Brazil-nut
effect," or why the first person to open a box of muesli
gets all the big pieces and the last helping contains only crumbled
oats. Theorists since the 1930s have blamed smaller grains for
slipping into the spaces created beneath larger particles. Others
claim that everything rises when shaken but only the smaller bits
find room to descend. The Chicago physicists suggested that we
can no longer simply blame the little guys; the problem is far
too complex.
Not
only must grains, nuts, and fruit be considered, Nagel and his
colleagues suggest, but also the air between particles. "Our
results," they conclude, "indicate an intricate interplay
between vibration-induced convection and fluidization, drag by
interstitial air, and intruder motion." In other words, both
the smaller particles and the air between particles act like fluids,
so variations of air pressure within the box alter how the nuts
"float." Despite this discovery, no one has yet developed
a pressurized cereal box.

So
pull out a napkin and wipe up the morning mess-the syrup, the
sugar, the stains. Then crush the napkin, compress it. Squeeze
as hard as you can.
It's
still 75 percent air. Nagel knows why.
Contributing
editor John Easton, AM'77, most recently wrote "Consuming
Interests" (August/01).

![]()
 Breakfast
is a messy meal. It involves sticky stuff that drips, like honey
or syrup, intended for pancakes but destined for your elbow, gritty
granules of sugar that cascade off your quivering spoon, and hot
caffeinated liquids that slosh, spill, and stain. Don't blame
yourself. Breakfast food, we now know, does not behave properly.
Coffee and a roll, hash browns, over-easy eggs, sausage, and a
side of toast: these are nonlinear and disordered phenomena, appearing
in macroscopic systems far from equilibrium.
Breakfast
is a messy meal. It involves sticky stuff that drips, like honey
or syrup, intended for pancakes but destined for your elbow, gritty
granules of sugar that cascade off your quivering spoon, and hot
caffeinated liquids that slosh, spill, and stain. Don't blame
yourself. Breakfast food, we now know, does not behave properly.
Coffee and a roll, hash browns, over-easy eggs, sausage, and a
side of toast: these are nonlinear and disordered phenomena, appearing
in macroscopic systems far from equilibrium. 

