Alumni
newsmaker:
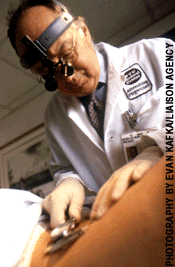
Jean-Claude Bystryn, SB'58
 Jean-Claude
Bystryn, SB'58, is trying to stop melanomas with a vaccine.
For the past five years, Jean-Claude Bystryn, SB'58, has focused
his cancer research on an experimental vaccine for melanoma, a
deadly skin cancer diagnosed in 45,000 Americans in 1999 alone.
The use of such vaccines in the war against cancer--now regarded
as revolutionary--was once controversial.
Jean-Claude
Bystryn, SB'58, is trying to stop melanomas with a vaccine.
For the past five years, Jean-Claude Bystryn, SB'58, has focused
his cancer research on an experimental vaccine for melanoma, a
deadly skin cancer diagnosed in 45,000 Americans in 1999 alone.
The use of such vaccines in the war against cancer--now regarded
as revolutionary--was once controversial.
"When
we first started working with this, nobody was thinking of treating
cancer with vaccines," Bystryn says. "It was considered black
magic. I was advised by many colleagues, 'When you talk about
what you do, don't use the word 'vaccine,' because people are
going to think you've gone off your rocker.'"
 Despite
the initial skepticism, Bystryn's vaccine has now reached the
final stage of testing--a large-scale clinical trial of 600 to
800 patients over the next four years--before approval by the
Food and Drug Administration (FDA) for use in melanoma patients
nationwide. The National Cancer Institute has agreed to produce
the vaccine for the large-scale testing. Meanwhile, Bystryn is
recruiting medical centers to participate in the trials and working
to secure funding.
Despite
the initial skepticism, Bystryn's vaccine has now reached the
final stage of testing--a large-scale clinical trial of 600 to
800 patients over the next four years--before approval by the
Food and Drug Administration (FDA) for use in melanoma patients
nationwide. The National Cancer Institute has agreed to produce
the vaccine for the large-scale testing. Meanwhile, Bystryn is
recruiting medical centers to participate in the trials and working
to secure funding.
To
create his vaccine, the New York University dermatologist, who
received his M.D. from NYU, isolates potential antigens--the elements
in the cancer cell that differ from the components of a healthy
cell. Then he injects them into the body in potent doses, building
up the immune system to block the actual antigens of the cancer
cells and destroy them.
"With
cancers, where the disease progresses slowly, there's a lot of
time available to build up your immune responses to fight against
the cancer," Bystryn explains, "and so the vaccine can be given
after you have the disease to boost the body's ability to fight
against the person's own cancer."
So
far, Bystryn has concentrated on treating high-risk cancer patients--those
with a 40 percent or more chance of fatality--whose cancer has
not yet progressed to the point of overtaking the entire immune
system. But his long-term goal is to develop the vaccine to be
safe and effective enough to administer to patients before they
develop melanoma, thus preventing the disease all together.
After
treating close to 700 melanoma patients in preliminary clinical
trials, Bystryn is optimistic. Initial trials showed a 50 percent
improved survival rate over the standard treatments, surgery and
chemotherapy.
But
the vaccine can't be approved by the Food and Drug Administration
for at least six years, and Bystryn worries about the patients
he cares for on a daily basis.
"There's
a pleasure and immediacy of taking care of people that you just
don't get doing basic research. It makes you appreciate the urgency,
the need, and the difficulties of doing work in man," the physician
says. "People who just do research sometimes get lost in the mice
and the test tubes, and they lose the reality check that you need
when you work with people. With medicine, the goal in the ultimate
is to help."
--B.B.


![]()
 Jean-Claude
Bystryn, SB'58, is trying to stop melanomas with a vaccine.
For the past five years, Jean-Claude Bystryn, SB'58, has focused
his cancer research on an experimental vaccine for melanoma, a
deadly skin cancer diagnosed in 45,000 Americans in 1999 alone.
The use of such vaccines in the war against cancer--now regarded
as revolutionary--was once controversial.
Jean-Claude
Bystryn, SB'58, is trying to stop melanomas with a vaccine.
For the past five years, Jean-Claude Bystryn, SB'58, has focused
his cancer research on an experimental vaccine for melanoma, a
deadly skin cancer diagnosed in 45,000 Americans in 1999 alone.
The use of such vaccines in the war against cancer--now regarded
as revolutionary--was once controversial.  Despite
the initial skepticism, Bystryn's vaccine has now reached the
final stage of testing--a large-scale clinical trial of 600 to
800 patients over the next four years--before approval by the
Food and Drug Administration (FDA) for use in melanoma patients
nationwide. The National Cancer Institute has agreed to produce
the vaccine for the large-scale testing. Meanwhile, Bystryn is
recruiting medical centers to participate in the trials and working
to secure funding.
Despite
the initial skepticism, Bystryn's vaccine has now reached the
final stage of testing--a large-scale clinical trial of 600 to
800 patients over the next four years--before approval by the
Food and Drug Administration (FDA) for use in melanoma patients
nationwide. The National Cancer Institute has agreed to produce
the vaccine for the large-scale testing. Meanwhile, Bystryn is
recruiting medical centers to participate in the trials and working
to secure funding.