|
||
      |
Brain waves
Olfaction researcher Leslie Kay explores what a rat’s sense of smell can teach us about how the brain learns
In the seventh grade Leslie Kay’s teachers gave her a troubling choice: she could take honors math, but only if she was “serious,” they said, because “there were a lot of boys who wanted to be in this program.”
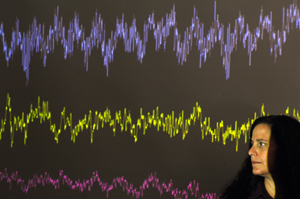
Leslie Kay measures the electrical pulses emanating from nerve cells in the brain’s olfactory bulb to study how rats discriminate between similar smells.
Kay, who at the time had no intention of going into science or math, went home and agonized over the decision. She wanted to take the math course—she thought it would be fun. She also did not appreciate her teachers’ implication about her gender. “I went back and lied to them,” she says, about her commitment to math.
An assistant professor of psychology at Chicago since 2000 and a member of the committees on neurobiology and computational neuroscience, Kay is an olfaction researcher. She leads a research team that has discovered how the brain automatically ramps up its activity to help mammals make a different kind of choice: fine distinctions between smells. In the August Journal of Neuroscience, the team reported the first direct measurements of how, as the subtlety of an olfactory decision increases, the brain’s olfactory bulb intensifies coordinated neural activity. In addition, by slightly changing rats’ behavioral response to a choice between two smells, the investigators discovered an unexpected and significant disparity in the neural circuitry required. This massive difference may shed new light, Kay says, on how the brain learns.
Though Kay had no plans to pursue research when she started college, her devotion to multidisciplinary education pushed her toward exactly that future. After earning a liberal-arts bachelor’s degree in 1983 from St. John’s College in New Mexico, she spent three years in Los Alamos with GenBank, a forerunner of the Human Genome Project. In 1985 she began a biophysics PhD at the University of California, Berkeley, that she interrupted after a year, traveling and working as a programmer for five years before finishing the degree in 1995. When she returned to Berkeley, she says, “I was more of a mathematician” than a scientist. She recalls walking into neurobiologist Walter Freeman’s lab and saying, “I want to do modeling”—using computers to simulate and understand natural processes. One of the tougher modeling jobs Freeman had on hand was that of the olfactory system’s dynamic. Kay soon realized that the existing data weren’t adequate; to make models, she’d have to do some experiments. Since the 1950s, smell researchers have known that the brain’s olfactory bulb—the central routing station between the nasal cavity and the higher brain—does not passively await incoming odor signals. Rather, it quivers in anticipation.
Inhalation itself excites the olfactory bulb into what’s called a “theta wave”: the bulb’s nerve cells vibrate electrically, tweaked by each breath into a state of receptivity that may increase sensitivity to faint signals. An oscillation of electrical pulses between nerve cells ranging from two to ten cycles per second, the bulb’s surveillance is Zen-like, focusing on no smells and all smells at once.
But that oscillation pattern—theta waves triggered by inhalation—changes when mammals try to differentiate one smell from another. Then their olfactory bulbs ring at a higher frequency. Gamma oscillations of 40 to 100 cycles emerge, for example, when rats and mice compare scents. Another oscillation pattern, 15- to 30-cycle beta waves, begins when the animal is about to identify a smell.
But experiments over the past several decades still left a lot of loose ends. Some didn’t test both brain activity and the resulting response behaviors, such as pressing or not pressing a bar, at the same time. Some involved anesthetized animals, whose neural activity may have been affected by the drugs. Others used mutated animals with dysfunctions outside the olfactory system. Perhaps most importantly, Kay says, past researchers used an experimental design in which the test animals had a “go/no-go” choice involving a punishment for making the wrong selection: the rat would press a bar to get a food reward for one scent; for the other scent, it would refrain from pressing the bar to avoid the punishment.
A test that factored out the differing reinforcements for different actions, Kay reasoned, would separate the olfactory choice from the behavioral one, thereby illuminating more precisely the neural architecture involved in odor discrimination. With sixth-year psychology grad student Jennifer Beshel and Boston University mathematician Nancy Kopell—who analyzed the experiments’ electrode records—Kay set out to observe alert rats asked to choose between two smells of varying similarity. In her team’s experiments, conducted in 2006, the rats needed to press one bar for one scent and another bar for the other scent, getting a reward for either correct choice. The tests showed that the rats used gamma waves to distinguish between odors, with finer distinctions requiring more intense gamma activity. But—in what Kay calls a “completely unexpected result”—the team detected no sign of the beta waves that previous research had said would precede a correct choice.
Kay suspects that the different results may have to do with the additional brain architecture recruited by beta versus gamma oscillations. Gamma waves are restricted to the olfactory bulb. Beta waves, meanwhile, also involve many other brain structures, including the piriform cortex, which analyzes smells; the hippocampus, which handles fact-based memories; and the entorhinal cortex, which feeds signals to the hippocampus and lights up during tasks that draw on memory. Kay reasons that a test like hers, which involves an artificial choice between two actions rather than a decision to act or not to act, might not elicit the same beta oscillations associated with that behavioral choice.
“The go/no-go task is probably ethologically more natural,” Kay says, meaning it comes closer to the choices animals make in the wild: eat or don’t eat; run or don’t run. The brain may simply be evolutionarily primed to solve that sort of problem. “Go/no-go is, in fact, easier,” she says. “The rats learn very quickly each new odor set.” But in Kay’s experiments—which require the rat to choose between two similar actions in response to two smells—it can take hundreds of trials for the rats to learn to discriminate between a single pair of odors.
That revelation points to perhaps the most interesting implication to emerge from synthesizing Kay’s findings with previous research, she says: earlier studies had shown that in beta tasks (which draw on a broader array of neural structures and which rats master more quickly) more information returns to the “sensors” in the olfactory bulb and the nasal cavity than they send on to the higher brain. The brain, she hypothesizes, may reprogram its sensors faster than they give it information. “Maybe that’s what makes the mammalian brain so good at learning,” Kay says. “It can use [its higher functions]”—not only the olfactory bulb, but also many other brain structures—“to inform the process.”
Beyond the puzzle of odor discrimination, Kay’s research has potential medical implications. Both the sense of smell and its associated brain areas are hit hard—and early—in Alzheimer’s, Parkinson’s, and Huntington’s diseases, as well as in schizophrenia. Basic research on smell, then, may help open a window into these dysfunctions, since studying a disease’s early steps often brings researchers closer to its cause.