|
||
      |
Blood work
One problem with studying the life-saving system of hemostasis is that roughly 80 known chemical reactions occur in the cascade of events that make blood clot. And although scientists understand what happens during many of those reactions, they know less about the network as a whole.
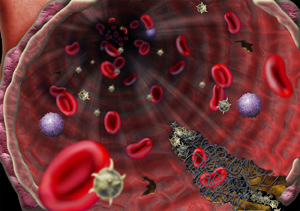
To prevent a clot from blocking a blood vessel, hemostasis occurs only at large sites of vascular damage.
So Chicago chemist Rustem Ismagilov and a team of graduate students sought to simplify the clotting system to find out why the body activates it for some injuries but not for others. To investigate the complex rules that control hemostasis, Ismagilov turned last year to microfluidics technology, which can mimic vascular clotting on the micron scale, a unit of measurement 100 times smaller than a human hair.
First, though, Ismagilov built a chemical model. Substituting nonbiological substances for blood and tissue factor—the clot-triggering protein released by damaged tissue—he also simplified hemostasis itself, focusing on only the three sets of reactions that simulate the major stages of the process: producing chemicals to activate clotting, inhibiting them to keep clotting under control, and, finally, forming the clot itself. “If you group reactions into modules and then think about one module at a time,” says Ismagilov, an associate professor of chemistry who joined Chicago’s faculty in 2001, “it’s much easier to understand.”
The strategy worked. Ismagilov’s research has yielded a major insight into the elaborate network of reactions, namely that “if you have several small damaged areas, you don’t get clotting,” he says, but if damage is concentrated in a larger area, blood does indeed begin to coagulate. “This appears to be a safety mechanism,” Ismagilov suggests, a way of making sure blood doesn’t clot where a clot isn’t needed—and where it might, in fact, impede circulation. A precisely tuned coagulation system, after all, can mean the difference between life or death. “Clotting has to occur at the right place at the right time,” he says. “A strong, rapid clotting response is essential to stop bleeding at a wound.” But if the damage is minuscule, a clot “can block blood vessels and can be life-threatening.”
The distribution of tissue factor—not the amount—governs the body’s hemostatic response. That’s the “punch line,” says Ismagilov, a Russian Academy of Sciences graduate who earned a chemistry PhD from the University of Wisconsin in 1998. If tissue factor is spread out across many tiny vessel breaks, no clot will form. The same quantity of tissue factor concentrated at the site of a bigger injury, however, triggers coagulation.
Ismagilov and his team found that simulated injuries measuring at least 100 microns always initiated hemostasis. When the researchers cut the injury’s diameter in half, however, nothing happened. Using microfluidics to test those results with real human plasma, Ismagilov’s team showed “that they pan out,” he says. Last October he and his research team documented their findings in the Proceedings of the National Academy of Sciences, and the work garnered Ismagilov the first Journal of Physical Organic Chemistry award for Early Excellence in Organic Chemistry.
The chemicals that activate clotting and those that inhibit overproduction of clots are always present in the body. But when a person suffers a large enough wound, say by falling off a bicycle, hemostasis activators are produced faster than the chemicals that keep the process in check. “When you produce enough activators,” says graduate student and research team member Christian Kastrup, “you get clotting.”
After the body coagulates enough blood to close the wound, “there’s a mechanism that re-moves the excess activator which promotes clotting. That allows the inhibitors to stop the clotting process.”
Ismagilov and colleagues previously developed a microfluidics method for crystallizing proteins, which aids drug development by allowing researchers to run hundreds of fine-tuned protein-crystallization tests with little manual labor. In the clotting experiment, the researchers used microfluidics to reproduce the tiny channels in the human network of veins, rather than having to rely, as scientists previously did, on flasks filled with homogeneous mixtures. The circulatory system is not so uniform, says Kastrup, adding that “microfluidics allowed us to mimic the surfaces and fluid environment of blood vessels.”
This was a critical step. “If we changed the geometry to something that didn’t look like a biological system,” says Ismagilov, “the [artificial] chemical system couldn’t function.”
Kastrup expects the same approach would work for researching other biological systems, such as those that control memory and decision making, or the growth of blood vessels. “If you can understand how they work, and how to predict their behavior,” said Kastrup, “you are on your way to being able to repair them when they are broken.”