|
||
      |
Features ::
Pipe Dream or Paradigm Shift?
You say bubbles, he says biocompatible surfactant—let’s call the whole thing off? For 15 years Chicago biochemist Raphael Lee has worked to bring a revolutionary therapy to trauma Patients.
Sometime back in 1990, Raphael Lee squinted through a microscope and watched as a slippery synthetic compound called poloxamer-188 stirred up a revolution. The ensuing odyssey would become political as well as scientific, but first there was simply an empirical discovery: a hypothesis and an experiment. In his surgery lab Lee had isolated a culture of rat muscle cells and delivered a deep, destructive electric shock. Vital intracellular material began gushing from tiny holes in the cell membranes. Then Lee doused the culture with poloxamer-188. Racing across cells ravaged by electricity, the compound sought out and mended the holes. Instead of dying, the tissue healed.
Two years later Lee, a surgeon and biomedical engineer who directs the University’s Program for Research in Molecular Repair, had conducted a host of similar experiments, with equally promising results. That May he published an account of his findings in Proceedings of the National Academy of Sciences, arguing that poloxamer-188 helps repair traumatized cells by resealing their perforated membranes and restoring the cells’ basic wholeness. In 1995 the Food and Drug Administration gave Lee the green light to begin testing poloxamer-188 (P188 for short) in human victims of electrical shock.
And then, nothing. More than a decade after winning FDA approval for clinical trials, Lee has yet to administer P188 to a single patient. When it first appeared, his research provoked skepticism from other physicians. Instead of directing shock victims toward Lee’s clinical trials, they steered patients elsewhere, and his increasingly comprehensive findings—and those of colleagues he enlisted to help flesh out the research—failed to gain much traction in the emergency room. The notion that physicians could rejuvenate critically damaged tissue instead of having to remove it was, Lee supposes, simply too new. Until a couple of years ago, when P188 research finally reached a persuasive critical mass, Lee says, “there was a lot of resistance in the burn community, even in our own burn community at the University of Chicago.” Outside trauma physicians “thought it was just a gimmick. It seemed impossible to people that you could apply a polymer and reverse trauma.”
Yet the roots of Lee’s far-fetched idea were planted in orthodox soil. “Protein chemists have known for decades that it’s possible, under very controlled laboratory circumstances, to restore damaged proteins and damaged membranes,” he says. What’s more, P188 is hardly exotic. A commercially available compound, it has long served as an emulsifier in blood products and drugs. “They’ve been using it in blood banks for 40 years.”
Lee’s brainstorm linking P188 to cell membranes came a few years after he began investigating precisely how electric shock kills tissues. “We figured out that both heat- and non-heat–related damage was involved,” Lee says, “and that the electricity was destroying the integrity of the cell membrane” by poking holes in it. Only about ten millionths of one millimeter across, membranes regulate traffic in and out of cells and protect the organic systems within them from the alien chemical environment outside. Any puncture in the wall can trigger a catastrophic chain of events: an influx of calcium ions, malformation of intracellular proteins, depletion of cell contents. Unrepaired, the cell will swiftly shrink and die. Knitting the membrane back together, however, gives the cell a chance to heal. “And so, of course, because we’re physicians, we looked for therapies,” Lee says. “We said, ‘How do you seal the membrane?’”
Here Lee found himself in uncharted territory. No researchers had written papers on the mechanics of cell-membrane repair. Instead, he says, they’d conjured “vague ideas about things like fusing membranes together.” In 1989 Lee became a surgery professor at the University. He’d been living in Boston, splitting his time between faculty appointments at Harvard and the Massachusetts Institute of Technology (where, as a research scientist, he won a 1981 MacArthur “genius” fellowship). Sometime during the family’s move to Hyde Park, Lee’s five-year-old daughter opened up a bottle of Mr. Bubbles bubble solution. Lee’s mind flashed.
“It had one of those plastic loops that you stick in the bottle,” he recalls, “and when you pull it out, there’s a thin film across it that you blow into to make a bubble. That’s when it occurred to me that what we needed to repair the cell membrane was a biocompatible surfactant.”
Surfactants are everywhere. They constitute the active ingredients in soaps and detergents—and bubble solutions. They show up in glue, fertilizers, herbicides, laxatives, lubricants, cosmetics, ink, and paint. Possessing both a hydrophilic component, which water attracts, and a hydrophobic component, which water repels, surfactants reduce surface tension. Oil, for instance, is a surfactant. Poured into a pot of water or a pond, oil coats the water and reduces the surface tension between it and the air.
Lee chose the surfactant P188 for its abundance, its nontoxicity, and its shape. A hydrophobic middle sandwiched between two hydrophilic ends, P188 mimics the composition of the cell membrane’s lipid bilayer, in which water-loving heads line the exterior and interior walls, isolating water-repellent tails at the membrane’s center.

Lee (holding a petri dish) believes similar therapies can help heal myriad traumatic injuries.
“The poloxamer won’t go into a normal cell,” says Chicago chemist and professor Ka Yee Lee (no relation), loading onto her laptop a colleague’s animated simulation of P188’s effect on ruptured membranes. She began collaborating with Raphael Lee in 1999, when he was recruiting chemists to help decipher the healing process. Her computer screen blinks and a full-color illustration appears, showing a lipid bilayer broken in several places. When she presses a command key, a handful of P188 molecules pop into view, veering purposefully toward gaps in the cell wall. The surfactant’s hydrophobic center buries itself in the membrane’s hydrophobic tail region, filling in the open spaces and forcing the lipids to cram closer together until the membrane becomes a solid wall, its “barrier function” restored.
The surfactant “only goes into cells whose integrity is damaged,” says Ka Yee Lee, “where the lipid packing density is reduced,” meaning the lipid bilayer is perforated and more loosely formed than it should be. “That’s good; it means the polymer [P188] is selecting out the cells that need repair. ... And once it’s present, it increases the packing density immediately.” Raphael Lee’s experiments indicate that if cells are treated within six to eight hours of an injury, P188 can reverse up to 77.4 percent of shock-induced trauma. When P188 is administered before the injury—a rare circumstance in the real world—the percentage rises to 96.3.
Besides acting as a “plug,” P188 changes the watery environment outside the cell, easing the surface tension so that the molecules disperse more easily. “The polymer breaks up the water so that it doesn’t stick to the membrane in a way that’s bad,” Raphael Lee says. “That’s a key part of the story.”
Once the breach closes and the cell starts returning to health, the membrane’s rising density simply squeezes out P188. “Where do you go when the bus gets too crowded?” Ka Yee Lee says. “You get pushed toward the door and out onto the street. The poloxamer goes to the surface and out of the cell. You don’t want [it] to act as a third wheel.”
In the past five years, Ka Yee Lee and other researchers have copiously described P188’s behavior within the cell membrane, not only in animal cells but also in isolated human cells and model cell membrane systems. In 1992, however, when Raphael Lee first published the results of his rat-cell experiments, no one had given a definitive explanation of how P188 worked. Consequently, many scientists weren’t convinced it did. He remembers an early audience with Chicago medical sages convened by Samuel Hellman, then dean of the Pritzker School of Medicine. “These were smart old guys,” Lee says, “who looked at new ideas” and decided which to give their influential support. Lee was 40 when he arrived at the University. “I took my bottle of Mr. Bubbles in there, and I showed it to them,” he recalls. “I don’t think they believed me at all.”
At first the blank stares, incredulous questions, and inattention Lee got from other physicians surprised him. “That’s one thing science and medical education doesn’t prepare scientists for—what to expect when you make a paradigm-shifting discovery,” he says, “what happens when you find something that challenges the way people think about things.”
But reflexive recalcitrance wasn’t the only fuel for skepticism. In clinical trials conducted in the early 1990s by researchers independent of Lee, P188 had proved toxic to some heart-attack patients. The problem, Lee says, arose from a misunderstanding. Believing that the poloxamer operated by facilitating blood flow to damaged tissue, some researchers advised physicians to administer much higher doses than turned out to be necessary. “They were giving hundreds of times the amount of the polymer over a sustained time than we find required to seal the membrane,” Lee says. “We give a bolus”—a small intravenous dose—“and then later another bolus. But if you think the polymer is necessary to keep the artery open—that that’s how it works—then you give it for days.” Like most anything else, in very high quantities P188 can turn toxic.
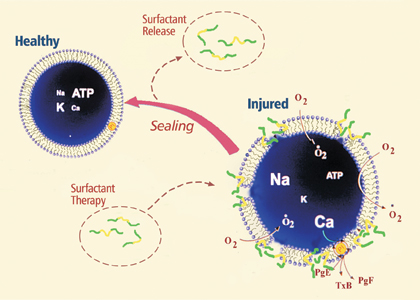
Poloxamer-188 works in part by sealing the holes in damaged cell membranes.
Courtesy Raphael Lee
Money shortages also plagued Lee’s hoped-for clinical trials. The National Institutes of Health wouldn’t foot the bill because testing P188 on electric-shock patients doesn’t fall into a fundable category. And the idea of spending millions of dollars to develop a treatment for an injury that affects only 2,000 or 3,000 people every year failed to entice pharmaceutical companies. “After all, they have shareholders too,” Lee says. The Electric Power Research Institute contributed a few hundred thousand dollars, but it was not enough to cover clinical-trial costs.
Even without patients, Lee marched on. Hypothesizing that if P188 could reverse electric-shock damage it might also undo other membrane-puncturing trauma, he organized experiments involving brain injury, spinal-cord damage, stroke, heart attack, and radiation exposure. Those tests are in various stages of inquiry and publication, but so far the results bolster his previous research. “Logically,” he says, “the polymer has the ability to assist with any injury or illness that involves membrane trauma.” He also is exploring the efficacy of P188 in reversing heart damage caused by defibrillators. “When somebody has a heart attack, you shock the heart to reset it,” Lee says, but that shock also causes injury. “It punches holes in the membranes. And after you do this and the heart returns to a bad rhythm, you have to shock it again. After a while, the heart loses its ability to reseal the membranes.” Several years ago Lee began probing a separate but related question: surfactants’ power to refold proteins structurally distorted by heat or other injury. The chemical principle is the same, he says. “Exposed hydrophobic parts of the protein stick together, and it’s hard to break them apart.” By reducing the surface tension and drawing in water with its hydrophilic head, a surfactant can relax the attraction between protein molecules. Unstuck, the protein can “refold back to its natural state.”
Increasingly, other Chicago researchers have joined ranks with Lee or embarked on their own P188 studies. Pediatrician and neurobiologist Jeremy Marks is investigating its effectiveness on cerebral palsy and brain injury. “Right now there are no medicines to save dying brain cells,” Marks says. “The more we know about the brain, the more mechanisms we can try to interfere with. But every time we tried, it’s either ineffective or there are intolerable side effects. The road to brain therapy is littered with failed attempts.” Lee’s discovery, however, led Marks and his colleagues to focus on the final chapter of a brain cell’s complex death: the breakdown of the cell membrane. “This therapy is different,” he says. “It doesn’t deal with individual mechanisms in any way. If you take the poloxamer and prevent that last step, you can give the cell enough time before it runs out of energy and dies to embark on repair.”
Marks’s experiments have proven productive. “In the dish I’ve
been able to save neurons by giving treatment as late as eight hours after
injury,” he says. His long-term goals are more ambitious. “My
research is babies, and so my interest here is in how quickly we can move
the polymer across the placenta, so that when babies are in distress during
labor, we can prevent things like mental retardation, deafness, and cerebral
palsy.”
Lee’s theories have found other converts as well. Researchers at Purdue
University, Boston University, Michigan State, the University of Toulouse,
and the University of Copenhagen have plunged into the study of P188. “The
bulk of the research is from the University of Chicago, but it’s not
just us now; it’s a whole field,” Lee says. “I’d
say we turned the corner about two years ago. We’re no longer out
in the wilderness with this idea. Now it’s mainstream, cutting-edge
science.”
In 2003 a surge of excitement from scientists and pharmaceutical officials greeted the creation of Maroon Biotech Corporation, which Lee and other Chicago researchers founded to help bring P188-based therapies to market. Soon, Lee says, Maroon Biotech will ask for FDA permission to test P188 on patients who’ve suffered spinal-cord and brain injuries. Lee has kept FDA approval up to date for clinical trials involving electric shock, and Maroon Biotech plans to undertake those tests too. Company researchers currently are conducting preclinical studies, refining the P188 cocktail, a mixture of the poloxamer and other compounds aimed at heightening its efficacy. They’re also hunting for other surfactants—natural or artificial—that may work even better than P188.
“The goal is to find something more effective, or to tailor a particular therapeutic product to a particular injury,” says Millicent Firestone, a chemist in Argonne National Laboratory’s materials-science division who joined Lee’s P188 research four years ago. Working with artificial lipid bilayers and manipulated versions of P188, Firestone says the poloxamer’s basic three-part shape is “optimal. It binds to the membrane, but not too tight.” She’s hoping to find other poloxamers that bind even more reliably.
This past fall Lee debuted the Pritzker course Cell Injury, Repair, and Death, exploring new treatments like P188. Every desk in the classroom was occupied, and Lee expects to repeat the class in upcoming semesters. “When you do something profoundly innovative, as Raphael did,” says Marks, who chairs Maroon Biotech’s scientific advisory board, “it takes time to convince people that something novel and unexpected can have the dramatic effect that this approach does.”
Imagining a time when burn patients won’t need skin grafts and even the most severely damaged tissue can be salvaged, Lee says the past 15 years have taught him patience. “You can complain about the peer-review system and say that it’s hard—and it is hard, because controversy is always personal,” he says. “And you might say it’s a bad system, but I’m not sure that it is. There are still a lot more bad ideas than good ones. In academia people say, ‘Time will tell.’ And that’s right. If it’s a good idea then no matter what the controversy, it won’t go away; it’ll stick around and get applied. So if you’ve got a better system, let me know.”
With that, Lee turns to his crowded bookshelf and plucks down a thin volume. “Here’s one of my most recent publications,” he announces, peeling open the cover of the fall 2004 Perspectives in Biology and Medicine. At the back of the journal, behind weighty ruminations on evidence-based medicine, gene therapy, and the Osler-Cushing Covenant, Lee reviews the latest biography of Ignaz Semmelweis, who spent the last eight years of his life trying futilely to convince Hungarian and Austrian physicians that washing their hands before walking into the delivery room would help prevent childbed fever. Semmelweis finally suffered a nervous breakdown and died in 1865, two weeks after being committed to a mental hospital. Years would pass before Joseph Lister and Louis Pasteur proved Semmelweis right.
Lee smiles. “Look,” he says, “things could be worse.”