|
||
      |
Heartfelt question
This much Eric Svensson, assistant professor of cardiology, knows already: before an embryo can begin to grow, its heart must beat. In humans, fruit flies, fish, and frogs, the heart is the first organ to develop—“without it, you’re dead in the water”—and it must begin pumping even before it acquires chambers and arteries and atrioventricular valves. “The first step is to form a tube, like a rubber hose,” he says, “that pumps.”
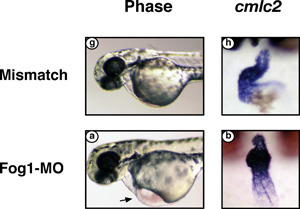
Deprived of FOG-1, a zebrafish’s embryonic heart swells with fluid
(bottom left) and never loops to form chambers (bottom right) the way
a normal heart does (top images).
After that, the course of cardiac development depends on the species, but for each it involves the same genes. No one’s sure exactly how many, but “I’ve heard the number 20,000 tossed around,” he says. He puts it in perspective: “There are 50,000 in an entire embryo.” Svensson, who earned a PhD in biochemistry and an MD from UCLA, focuses on two of those genes: FOG-1 and FOG-2 (as a Chicago postdoc, he identified the latter and began cloning it in 1997). Both are transcription factors, proteins that help regulate other genes’ expression. Svensson and his colleagues try to decipher their role in heart development, to figure out the interaction between FOG-1 and FOG-2 and other genes, in hopes of “understanding how the human heart is put together” and, eventually, understanding congenital heart disease.
In large part, their investigations involve laboratory animals—embryonic mice or zebrafish—deprived of FOG-1 or FOG-2. They study each embryo’s progress and compare the genes at work in normal hearts with those active in the altered ones. In 1999, for example, Svensson engineered a mouse to lack FOG-2. After 13 days, halfway through gestation, it died of what looked like heart failure: swelling and fluid under the skin and around the heart. “I take care of cardiology patients with heart failure who look just like this,” he says. “Their hearts don’t pump well enough to get rid of all the fluid they take in in a day, and so the fluid backs up into their lungs and they get short of breath; it backs up into their legs and they swell up.”
Looking closer at the mouse heart he discovered a number of problems. The heart tube emerged properly and then “looped,” or folded together to begin forming four chambers, “but it didn’t mature as it should have, so the mice ended up with a hole in their heart between the left and right ventricles,” says Svensson, who published the results in the July 2000 Nature Genetics. “They ended up with very thin-walled ventricles; normally they have to be thicker to pump the blood through the whole body.” Instead of forming two valves to connect the top and bottom chambers, the mice got one oversized valve that didn’t work. Their coronary arteries—the vessels that supply blood to the heart—never formed at all.
“This is very interesting,” Svensson says, especially as cardiologists search for novel ways to remedy coronary blockages, a major cause of heart attacks and the cardiac-tissue death that follows. Surgeons now treat the problem using stents and angioplasty, but these solutions are temporary; blockages invariably creep back. “There’s a lot of attention on figuring out how to actually regrow cardiomyocytes”—heart muscle cells—“and interest in using stem cells to do that,” he says. “So far that hasn’t worked—partly because we don’t fully understand how to make those heart cells.” Svensson’s mouse model shows that FOG-2 is critical in forming coronary arteries: “FOG-2 must tell the myocytes to make some kind of signal that causes these coronaries to form.” The next step is narrowing down the list of 800 or so genes that interact with FOG-2 and isolating the one or two or several that govern coronary formation. It is a process of elimination, of comparing gene expression in normal and altered hearts. “If you could regrow new coronaries, you could get around blockages without stents and continue to get blood to the heart.” A few patients do this naturally, developing what cardiologists call “collaterals” that bypass blockages by opening new connections between arteries. “That happens for reasons we don’t yet understand,” he says. “We’re trying to find that genetic signal, and then we’re off to the races.”
Recent experiments give Svensson reason to wonder whether FOG-1 may also be involved in coronary formation. In the January 2006 Developmental Biology, he described the progress of a zebrafish embryo lacking FOG-1—which he discovered to be the only FOG gene expressed in the species’s two-chambered heart. Like the mouse, the zebrafish didn’t survive beyond midgestation, but unlike the mouse, its heart never looped to begin forming chambers. “They didn’t even make that early step in development.” Fluid began collecting in the zebrafish embryo’s pericardial sac, and its ventricle walls never fully developed.
“Flipping back and forth between species helps us figure different things out,” says Svensson, who’s planning further mouse experiments and perhaps another using frog embryos. The zebrafish study raises a few crucial questions. “Do zebrafish form coronaries? We think they do—we think we have pictures of that—but is the FOG-1 gene important in regulating the formation of those coronaries? And if it is, does it work the same way? We don’t have the answer yet, but when we get it, we’ll know a lot more about human heart disease.”