|
 And...reAction! And...reAction!
As Titanic
captured the Oscar limelight, a much smaller, shorter action picture
opened to its own glowing reviews. In the March 20 issue of Science,
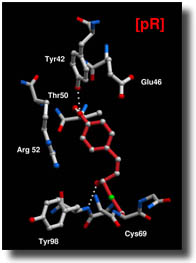
an international team led by Keith Moffat presented the first images
of how light gets transformed into chemical energy—the first stage
of activities as different, and fundamental, as photosynthesis and
vision.
Moffat is a
professor in biochemistry & molecular biology and executive director
of the University’s Consortium for Advanced Radiation Sources (CARS).
His research team included Benjamin Perman, SM’96, of biochemistry;
Vukica Srajer, Zhong Ren, and Tsui-yi Teng from CARS; and researchers
from the European Synchrotron Radiation Facility (ESRF) in Grenoble,
France; Lund University; and the E.C. Slater Institute of the University
of Amsterdam, Netherlands.
The team’s
images were made possible by a recent, million-fold improvement
in the time resolution of X-ray measurements at ESRF, letting researchers
produce pictures of changes in the shape of a working protein that
occur in billionths of seconds. The process, still being refined
by several teams worldwide, involves cooling the “actors” to slow
their activity while focusing extremely bright lights on them to
increase the camera’s shutter speed.
Unlike Leonardo
DiCaprio, however, the stars of Moffat’s pictures—in this case,
a blue-light photoreactive protein of the xanthopsin family’s eubacterium
Ectothiorhodospira halophila—have not become anything like a household
name.
The bacterium—which
is found only in a few high, arid lake beds in Oregon and salt depressions
in the Egyptian desert—is “rather obscure,” admits Moffat. “On second
thought, make that ‘incredibly obscure.’” But its status as an unknown
may soon be a thing of the past. Its light-sensitive protein’s debut
performance is bringing it to scientific center stage, attracting
attention not just from biochemists but also from computer engineers
who hope the protein may some day play a leading role in their projects,
such as the development of optical computers.
The star protein
has all the qualifications a casting director might select for an
optical storage and transport mechanism. When exposed to a light,
the protein immediately flips from one structure to a slightly different
conformation. “In the dark, the system is cocked and ready for structural
changes,” explains Moffat. A single photon provides enough energy
to “pull the trigger,” he says.
The protein
appealed to the research team because it is comparatively small,
simple, and water-soluble. Its charm for optical computing, on the
other hand, is that it is extremely robust. “If handled correctly
it can tolerate intense, repetitive stimulation from lasers, X-rays,
or light,” says Moffat.
Although the
Science paper focused only on the first billionth of a second
after light exposure, the research team has gathered information
on a series of subsequent changes in the molecular structure after
that first impulse. The end result will be a longer motion picture,
lasting several billionths of a second, that follows the subsequent
steps in this fundamental biological process.
The researchers
are also eager to perform still faster and more detailed crystallography
at the Advanced Photon Source at Argonne National Laboratory, permitting
them to observe biomolecules in their true, dynamic state.
Meanwhile,
the bacteria are responding in typical Hollywood fashion to all
the enormously bright lights beamed at their delicate photoreceptors:
Like starlets confronted by paparazzi, they swim the other way.—John
Easton
|



